Cdcl3 Nmr Peak

Cdcl3 Nmr Peak. Using chemical shifts, the peak at 1.2 ppm is in the expected range 3. Thus at a magnet strength of 1.41 tesla although hz are the fundamental energy unit of nmr spectroscopy, the use of hz has the disadvantage that the position of a peak is dependent on the magnetic field strength. H nmr chemical shifts for acetic acid (ch. Occa sion a lly, in or der t o dist in gu ish bet ween pea ks wh ose a ssign m en t wa s. I expected the peaks to have the normal splitting patterns, because the nmr mentions it was done in cdcl3 and this is not neat pyridine. Sh ow t h eir degr ee of va r ia bilit y. What is this peak due to and why the heck is it there? Most nmr spectra are recorded for compounds dissolved in a solvent. Peaks in the 13c nmr spectra corresponding to the deuterated solvent molecules show. Practical nmr as a side note: Using chemical shifts, the peak at 1.2 ppm is in the expected range 3.
From this spectrum we determined the chemical shifts of the solvent residual peak2 and the water peak. 1% is way too much for a high field instrument, imo. Whenever you run a #^13c# spectrum in cdcl₃, you always get a triplet solvent peak at 77.5 ppm. When comparing two nmr spectra, always keep in mind the subtle differences in the way the spectra were recorded. What is this peak due to???? However, whenever cdcl3 is used as an nmr solvent, a small singlet is always observed at 7.26 delta.
Practical nmr as a side note:
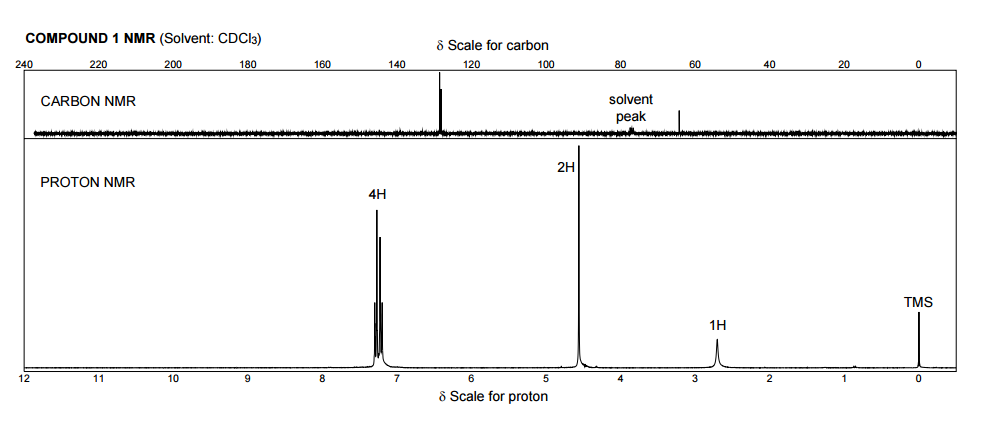
Figure nmr19.1h nmr spectrum of ethanol with normalized integral numbers. Multiplet structures from the 1h nmr spectrum of ethanol in cdcl3. As the bo field increases in magnitude (i.e. One obvious example is the effect of field strength. All of the peaks given are in reference to a standard for proton nmr. Unique or peculiar spin coupling patterns, making these especially for these reasons, the 13c nmr spectra of most ordinary organic compounds exhibit only singlet resonances for each carbon in the 13c nmr. Write down the new tof value after typing dg. Two peaks in a ratio of 1h:2h could correspond to one and two hydrogens, or they could correspond to two and four hydrogens, etc. That peak is corresponded to the relative small conc of chcl3, b/c chcl3 is one of the reagents that is used to make cdcl3. 1% is way too much for a high field instrument, imo. Since the 1d carbon experiment is highly susceptible to the 13c nuclei in the. What is this peak due to????
Most nmr spectra are recorded for compounds dissolved in a solvent. Start date jun 6, 2008. Your tms peak will be way too strong for most samples unless they are pretty concentrated, like in a c13 analysis.

Whenever you run a #^13c# spectrum in cdcl₃, you always get a triplet solvent peak at 77.5 ppm.
Occa sion a lly, in or der t o dist in gu ish bet ween pea ks wh ose a ssign m en t wa s. Put the cursor on a peak and type movetof. Start date jun 6, 2008. Practical nmr as a side note: What is this peak due to and why the heck is it there? Thus at a magnet strength of 1.41 tesla although hz are the fundamental energy unit of nmr spectroscopy, the use of hz has the disadvantage that the position of a peak is dependent on the magnetic field strength. However, whenever cdcl3 is used as an nmr solvent, a small singlet is always observed at 7.26 delta. Peak multiplicities are produced by vertical traces from peaks in the 2d spectrum, as indicated by green lines in figure 5, and help in determining the figure 7 shows the 13c nmr spectra of 2 m ibuprofen in cdcl3. Therefore, signals will be observed for the solvent and this must be accounted for in solving spectral problems. As the bo field increases in magnitude (i.e.
The larmour precession frequency νo depends on the magnetic field strength. Another group of peaks you can usually rule out is anything with a very small. Using chemical shifts, the peak at 1.2 ppm is in the expected range 3. Therefore, signals will be observed for the solvent and this must be accounted for in solving spectral problems. Since the 1d carbon experiment is highly susceptible to the 13c nuclei in the. The standard for chemical shift is dilute tetramethylsilane (tms) in cdcl3, but many measurements are made relative to tms in other solvents, the proton resonance of the solvent peak or relative to nmr measurements were recorded on a bruker drx 400 spectrometer (1h tms resonance 400.130 mhz).
H nmr chemical shifts for acetic acid (ch.
That peak is corresponded to the relative small conc of chcl3, b/c chcl3 is one of the reagents that is used to make cdcl3. Nuclear magnetic resonance spectroscopy, most commonly known as nmr spectroscopy or magnetic resonance spectroscopy (mrs). You are not allowed find the transmitter frequencies for each peaks as follows: It is like a little fraction. Proton nmr and carbon nmr tables aid chemists in separating signals of impurities that might originate from residual solvents or a reaction apparatus. However, whenever cdcl3 is used as an nmr solvent, a small singlet is always observed at 7.26 delta. Cdcl3 is a common solvent used for nmr analysis. Write down the new tof value after typing dg. Hydrogen atoms as little magnets. Proton 90° pulse width calibration. Cdcl3 is a common solvent used for nmr analysis. When the exchange rate between h20 and hdo is slow on the nmr timescale the water peak appears as two peaks, a singlet corresponding to h20 and a 1:1. Your tms peak will be way too strong for most samples unless they are pretty concentrated, like in a c13 analysis. In this video, i take a look at how to determine the number of peaks on a 1h nmr spectrum of an organic compound by looking at its structure formula. What is this peak due to and why the heck is it there?
Spectrum taken in cdcl3 on a varian gemini 2000 spectrometer with 300 mhz cdcl3. Since cdcl3 has 1 deuterium (n = 1), and the spin type is 1 (i = 1), you get 2(1)(1) + 1 = 3, so 3 peaks.
 Source: www.qorganica.es
Source: www.qorganica.es Proton nmr and carbon nmr tables aid chemists in separating signals of impurities that might originate from residual solvents or a reaction apparatus.
 Source: cloudfront.jove.com
Source: cloudfront.jove.com Since cdcl3 has 1 deuterium (n = 1), and the spin type is 1 (i = 1), you get 2(1)(1) + 1 = 3, so 3 peaks.
 Source: www.redalyc.org
Source: www.redalyc.org Proton 90° pulse width calibration.
 Source: 3.bp.blogspot.com
Source: 3.bp.blogspot.com One obvious example is the effect of field strength.
 Source: www.ncbi.nlm.nih.gov
Source: www.ncbi.nlm.nih.gov The larmour precession frequency νo depends on the magnetic field strength.
Your tms peak will be way too strong for most samples unless they are pretty concentrated, like in a c13 analysis.
 Source: www.sigmaaldrich.com
Source: www.sigmaaldrich.com What is this peak due to and why the heck is it there?
 Source: media.springernature.com
Source: media.springernature.com Practical nmr as a side note:
 Source: d3i71xaburhd42.cloudfront.net
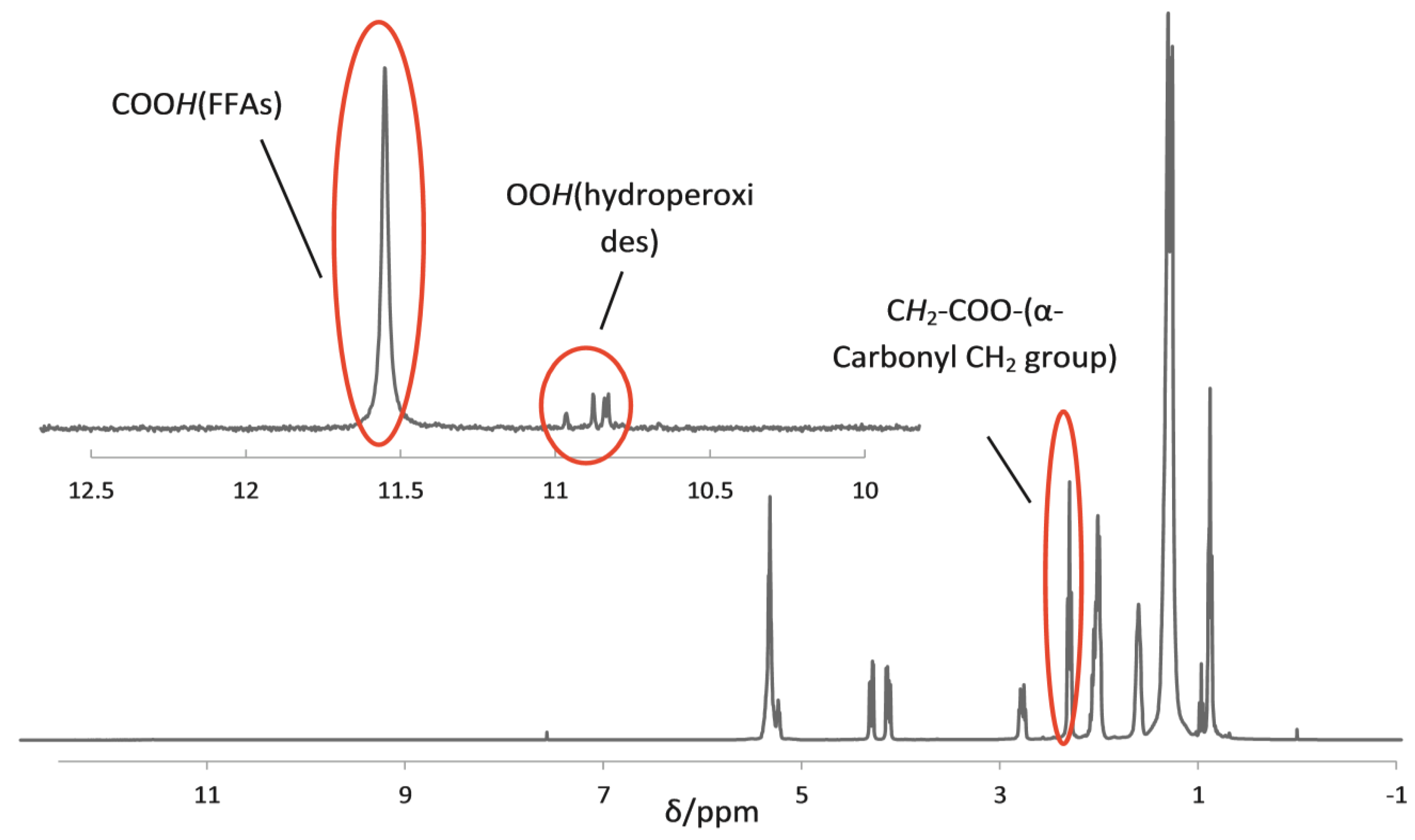
Source: d3i71xaburhd42.cloudfront.net Peak multiplicities are produced by vertical traces from peaks in the 2d spectrum, as indicated by green lines in figure 5, and help in determining the figure 7 shows the 13c nmr spectra of 2 m ibuprofen in cdcl3.
 Source: www.sigmaaldrich.com
Source: www.sigmaaldrich.com One obvious example is the effect of field strength.
 Source: 3.bp.blogspot.com
Source: 3.bp.blogspot.com Spectrum taken in cdcl3 on a varian gemini 2000 spectrometer with 300 mhz.
 Source: bmrb.io
Source: bmrb.io Using chemical shifts, the peak at 1.2 ppm is in the expected range 3.
 Source: chem.ch.huji.ac.il
Source: chem.ch.huji.ac.il Start date jun 6, 2008.
 Source: i.redd.it
Source: i.redd.it In this video, i take a look at how to determine the number of peaks on a 1h nmr spectrum of an organic compound by looking at its structure formula.
 Source: 4.bp.blogspot.com
Source: 4.bp.blogspot.com Therefore, signals will be observed for the solvent and this must be accounted for in solving spectral problems.
 Source: www.chemicalbook.com
Source: www.chemicalbook.com Sh ow t h eir degr ee of va r ia bilit y.
 Source: d2vlcm61l7u1fs.cloudfront.net
Source: d2vlcm61l7u1fs.cloudfront.net 1% is way too much for a high field instrument, imo.
 Source: study.com
Source: study.com Cdcl3 is a common solvent used for nmr analysis.
 Source: www.mdpi.com
Source: www.mdpi.com What is this peak due to????
 Source: study.com
Source: study.com Your tms peak will be way too strong for most samples unless they are pretty concentrated, like in a c13 analysis.
 Source: media.springernature.com
Source: media.springernature.com Another group of peaks you can usually rule out is anything with a very small.
 Source: www.qorganica.es
Source: www.qorganica.es Spectrum taken in cdcl3 on a varian gemini 2000 spectrometer with 300 mhz.
 Source: img.homeworklib.com
Source: img.homeworklib.com I expected the peaks to have the normal splitting patterns, because the nmr mentions it was done in cdcl3 and this is not neat pyridine.
 Source: 2.bp.blogspot.com
Source: 2.bp.blogspot.com Sh ow t h eir degr ee of va r ia bilit y.
 Source: d3i71xaburhd42.cloudfront.net
Source: d3i71xaburhd42.cloudfront.net Spectrum taken in cdcl3 on a varian gemini 2000 spectrometer with 300 mhz.
 Source: sites.science.oregonstate.edu
Source: sites.science.oregonstate.edu Write down the new tof value after typing dg.
 Source: employees.csbsju.edu
Source: employees.csbsju.edu From this spectrum we determined the chemical shifts of the solvent residual peak2 and the water peak.
When comparing two nmr spectra, always keep in mind the subtle differences in the way the spectra were recorded.
 Source: www.redalyc.org
Source: www.redalyc.org Cdcl3 is a common solvent used for nmr analysis.
 Source: www.spectralservice.de
Source: www.spectralservice.de When the exchange rate between h20 and hdo is slow on the nmr timescale the water peak appears as two peaks, a singlet corresponding to h20 and a 1:1.
Nuclear magnetic resonance is concerned with the magnetic properties of certain nuclei.
 Source: www.chemicalbook.com
Source: www.chemicalbook.com The thing is that i read somewhere that the peak should be at 7.24 ppm but there is a lot of peak in this region of my spectra so how can i determine where is it exactly.
 Source: employees.csbsju.edu
Source: employees.csbsju.edu Hydrogen atoms as little magnets.
 Source: ars.els-cdn.com
Source: ars.els-cdn.com However, whenever cdcl3 is used as an nmr solvent, a small singlet is always observed at 7.26 delta.
First of all let me clear that cdcl3 is not used always in recording the spectra.
.jpg) Source: d12oja0ew7x0i8.cloudfront.net
Source: d12oja0ew7x0i8.cloudfront.net Hydrogen atoms as little magnets.
 Source: www.ncbi.nlm.nih.gov
Source: www.ncbi.nlm.nih.gov That peak is corresponded to the relative small conc of chcl3, b/c chcl3 is one of the reagents that is used to make cdcl3.
 Source: sites.science.oregonstate.edu
Source: sites.science.oregonstate.edu All of the peaks given are in reference to a standard for proton nmr.
Posting Komentar untuk "Cdcl3 Nmr Peak"